BIOACID I – Theme 3: Calcification: Sensitivities across phyla and ecosystems
Overarching questions
- What are the cellular mechanisms of calcification and decalcification in different marine organisms, particularly with regard to ion transport to and from calcification sites?
- How will OA and pH stress affect calcification at the level of organisms, communities and ecosystems? Will concurrent temperature change modulate these effects?
- What changes will occur in the ultra-structure, trace element partitioning, and isotopic signature of the shells and skeletons of calcifyers in response to pH stress?
- How does the changing water column chemistry influence carbonate dissolution and deposition in sedimentary systems?
- Are past OA events (e.g. in the Cenozoic) useful analogues for projected future OA in their effects on calcifying organisms? Is the performance and sensitivity of past and present calcifyers comparable, and does this information help to assess the future?
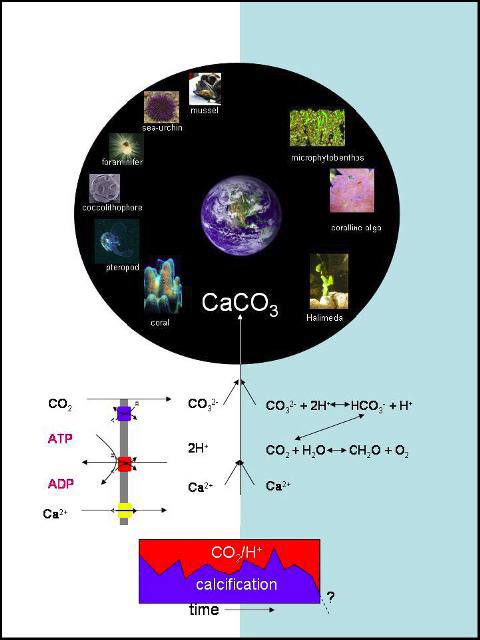
Calcification, the precipitation of calcium with carbonate, is influenced by the current increase in atmospheric CO2 levels and its concurrent gradual decrease in ocean pH. Since acidification leads to a decrease of the carbonate ion concentration, ocean acidification causes a decrease of the carbonate saturation sate. Hence we can expect a future reduction in calcification rates, or even net decalcification. Indeed reduction of calcification rates have been observed upon acidification in several marine calcifying groups, but not in all. This is indicative for a variety of calcification mechanisms. Calcification is a proton-generating process, and decalcification is proton consuming. Thus, these processes are typically coupled to either proton consuming (calcification) or proton producing (decalcification) processes. Conversely, calcification and decalcification can buffer other pH changing processes, and will thus to some extend buffer the oceanic pH.
Biological calcification always occurs in more or less isolated microenvironments, in which the carbonate and/or calcium concentrations are changed by biological activity. These changes can be driven by ion pumps in specialized transport tissue, e.g. for Ca2+ or H+, which is typical for the highly controlled calcification in corals, foraminifera, bivalves and coccolithophores. Alternatively, calcification can be a side effect of metabolic activities such as photosynthesis, occurring in a matrix with mass transfer limitation (a sediment or microbial mat). We will investigate if some calcification mechanisms are more sensitive than others, and the extent to which decalcification can buffer the decrease in oceanic pH. We will further investigate if decalcification can contribute to pH buffering of the oceans. We will finally investigate if the oceanic pH may leave signatures in the biogenic carbonates, and learn from acidic events in the past what the effect is on biodiversity on calcifying nano-plankton.