What is ocean acidification?
Since the beginning of the Industrial Revolution, the ocean has absorbed about 30 per cent of all the carbon dioxide (CO2) released into the atmosphere by human activities.
By providing this invaluable service – science refers to it as a “CO2 sink” function – the ocean slows down global climate change. If this natural store would not exist, our planet would heat up much more and much faster than we observe today. Carbon dioxide is a dangerous greenhouse gas: Once it gets into the atmosphere, it reflects heat radiated from the earth and it starts to heat up.
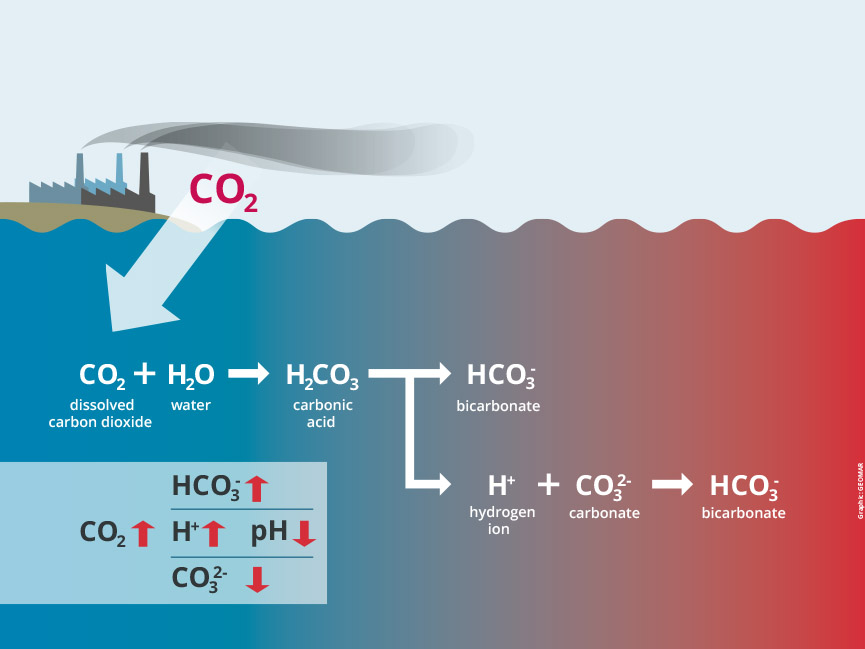
But in turn, as CO2 dissolves in the ocean, it triggers a chemical reaction with far-reaching consequences: carbonic acid is produced and the water becomes more acidic – its pH declines. Since the beginning of the Industrial Revolution, the average pH of the global ocean surface has already fallen from 8.2 to 8.1, corresponding to an increase in acidity of about 26 per cent.
As the ocean acidifies, the concentration of carbonate ions decreases. Calcifying organisms such as mussels, corals, and various plankton species need exactly these molecules to build their shells and skeletons. The less carbonate ions are available, the more costly calcification becomes. How much larger the additional effort will be exactly, depends also on the form of calcium carbonate the organisms produce – the more soluble aragonite or the steadier calcite.
Also other marine organisms that do not have calcium carbonate shells or skeletons need to spend more energy to regulate their bodily functions in acidifying waters. Additional energy that becomes necessary for survival under more acidified conditions will no longer be available for growth, reproduction, or resistance to other environmental stresses.
At the same time, some species, such as seagrass and blue-green algae, may benefit from the additional CO2 dissolved in seawater – there are winners and losers in the food web
For the process of ocean acidification, two chemical reactions are particularly important. They can occur simultaneously:
The formation of carbonic acid and the subsequent release of hydrogen ions:
CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3–
(carbon dioxide + water ↔ carbonic acid ↔ hydrogen ions + hydrogen carbonate ions)
The reaction between carbonate ions, CO2 and water, resulting in bicarbonate ions:
CO2 + H2O + CO32- 2↔ HCO3–
(carbon dioxide + water + carbonate ions ↔ bicarbonate ions)
LEARN MORE: Acidification in the Arctic // Plankton // Cold-water corals // Tropical corals // Society impacts